REGEND001 New Drug Clinically Accepted by CFDA
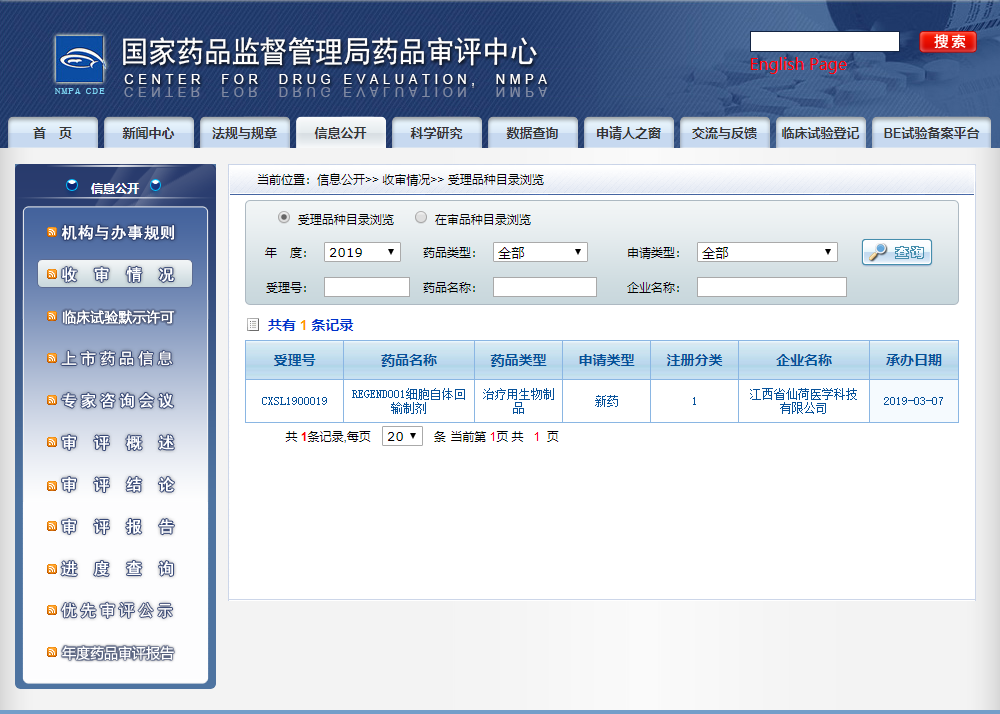
发布时间:19/07/01Recently, the application for clinical trial of REGEND001 cell autotransfusion preparation independently developed by Jiangxi Xlotus Medical Science and Technology Co., Ltd., the core platform of Regend Group, has been accepted by the Drug Evaluation Center of the State Drug Administration with the acceptance number CXSL1900019. REGEND001 cell preparation is the first autologous stem cell product for respiratory diseases in the world. It is also the first autologous stem cell product to help the respiratory system regenerate. It is also the first autologous stem cell product accepted by China.
Regend was founded in July 2015. The company's chief scientist is Professor Zuo Wei, a high-tech company focusing on human organ regeneration medicine. Ji Mei Ruisheng is the first enterprise in China that has been registered by the National Health and Health Commission and the Food and Drug Administration (FDA) and approved to carry out stem cell clinical research (registration number: CMR-20161214-1002); it is also the first enterprise to undertake the key research and development project "stem cell and transformation research" of the Ministry of Science and Technology. The company has patented technology for isolation and expansion of adult stem cells from epithelial tissue, which solves the worldwide problem of isolation and expansion of such cells. Some of the results have been published in the world's leading academic journal Nature and applied for a number of national patents. Some of the research results of "adult stem cells for lung tissue regeneration" have been published in the Journal Protein & Cell. The research progress has also been selected as the "Ten Progress in Chinese Medical Biotechnology" in 2018.

Based on many years of scientific research, Regend's self-developed REGEND001 cell autotransfusion preparation initiated a phase I/II open, historical controlled clinical study in early 2016. It preliminarily evaluated the safety and effectiveness of autologous bronchial basal layer cells in the treatment of COPD, bronchiectasis, interstitial lung disease and so on. In early 2018, clinical studies with the same period of control were launched, which has been compared with more than 20 countries in the country. Clinical research was carried out in collaboration with third-class A hospitals.
At present, injury and degenerative diseases are one of the main threats to human health in China. Traditional methods can not be completely cured, but can only delay the development of the disease. The reason is related to the lack of effective means to repair the structural damage of human organs. The new generation of adult stem cells in regenerative medicine can reproduce the development process and regenerate the body, thus becoming a new treatment method, which provides a new idea for the treatment of such diseases.
In order to improve the quality of medicines and promote the structural adjustment and transformation and upgrading of the pharmaceutical industry, the state has continuously introduced relevant incentive policies. In December 2017, CFDA issued the "Notice on Adjusting the Examination and Approval of Drug Clinical Trials (Draft for Consultation)", which stipulates the management measures for completing the examination and approval within the deadline, and again emphasizes the determination of the state to speed up the examination and approval of innovative drugs.
The clinical data of REGEND001 have been summarized and submitted, and the application for the first new autologous stem cell drug for lung diseases in China has been accepted by the Drug Administration.
Regend's application for clinical trials of new stem cell drugs is not only a summary of the research and development process in the past few years, but also a new start before the new drugs are put on the market. It will not only benefit a large number of patients in the future, but also create great social value for its industrialization demonstration.